What Is Phosphorus Oxychloride?
Phosphorus Oxychloride is a compound in which an oxygen atom is added to phosphorus trichloride.
It reacts violently with water, producing decomposition products including hydrogen chloride and phosphoric acid. In addition, moisture in the air produces hydrogen chloride gas.
It is possible to make phosphorus trichloride by reacting it with oxygen and oxidizing it directly. In addition, phosphorus pentachloride, which is made by the action of chlorine on phosphorus trichloride, can be produced by decomposing it with water. Phosphorus trichloride is obtained by reacting phosphorus with chlorine.
Uses of Phosphorus Oxychloride
Phosphorus Oxychloride is used in the manufacture of hydraulic fluids, plasticizers, gasoline additives, and fire retardants. It can also be used in the manufacture of pharmaceuticals, dyes, phosphorus pesticides, fragrances, raw materials for nonflammable films, and uranium ore extractants.
In addition, it is used as a diffusing agent in the semiconductor manufacturing process.
Properties of Phosphorus Oxychloride
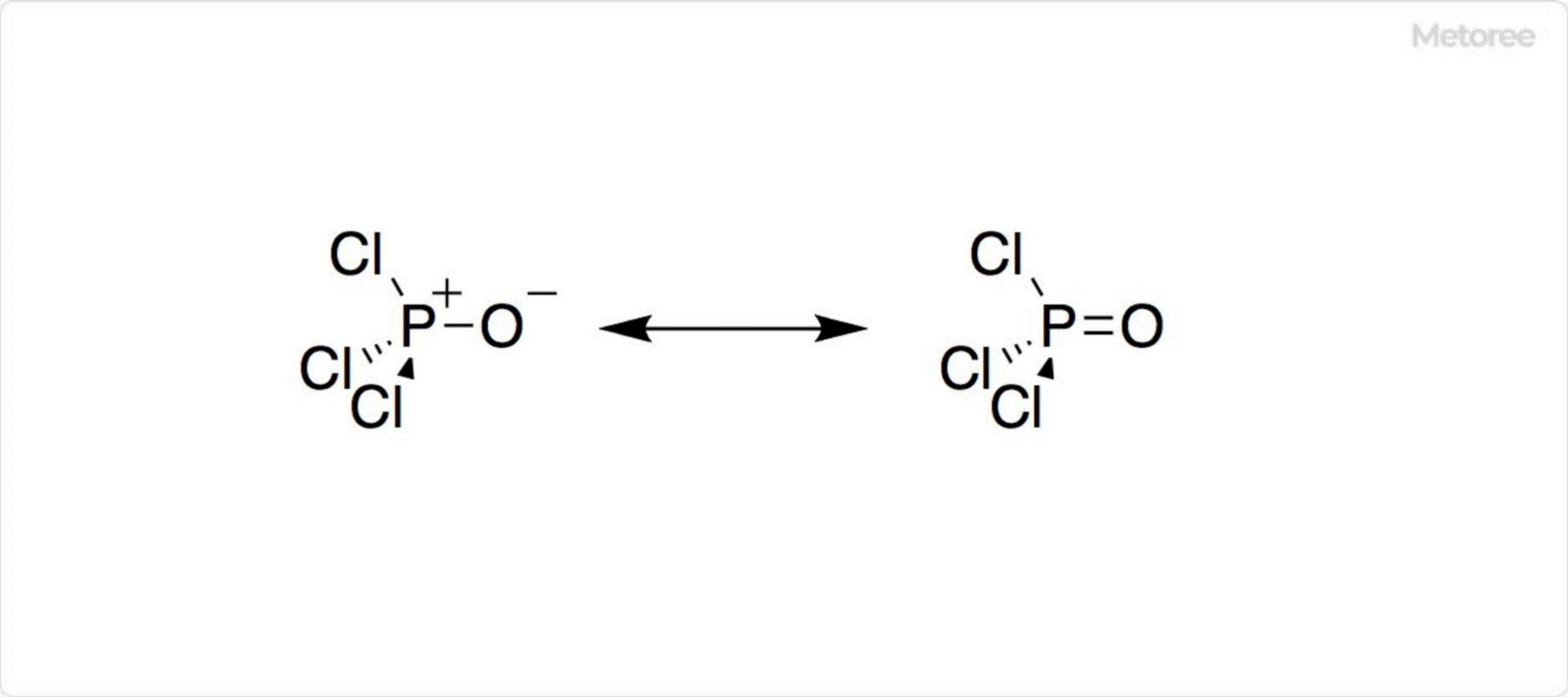
Figure 1. Structure of phosphorus oxychloride
Phosphorus Oxychloride has a melting point of 1.25°C and a boiling point of 105.8°C. It is a colorless, fuming liquid with a pungent odor. The vapor is heavier than air. Its molecular formula is POCl3 and its molar mass is 153.33 g/mol. It is also called phosphoryl chloride or trichloride phosphate
Phosphorus Oxychloride is a phosphorus-centered tetrahedral chemical with three P-Cl bonds and one P=O bond; the P=O bond is very strong, with a bond dissociation energy of 533.5 kJ/mol. The Schomaker-Stevenson rule states that the contribution of the double bond is considerably larger than that of phosphoryl fluoride (POF3) in terms of bond strength and electronegativity.
The P=O bond in phosphorus oxychloride is different from the π bond in the carbonyl group of ketones; the P-O π bond appears to result from the donation of a lone electron pair of an O atom to the σ* orbital of P-Cl. However, the P-O interaction is still long disputed.
Other Information on Phosphorus Oxychloride
1. Reactions of Phosphorus Oxychloride
Triaryl phosphates can be obtained by heating excess phenol and phosphorus oxychloride together with a Lewis acid such as magnesium chloride.
Phosphorus Oxychloride can also act as a Lewis base. For example, when reacted with a Lewis acid such as titanium tetrachloride, it produces an adduct.
The adduct of phosphorus oxychloride and aluminum chloride is stable, and aluminum chloride can be completely removed from the mixture after the Friedel-Crafts reaction. In the presence of aluminum chloride, phosphorus oxychloride reacts with hydrogen bromide to give POBr3.
2. Reaction With Phosphorus Oxychloride
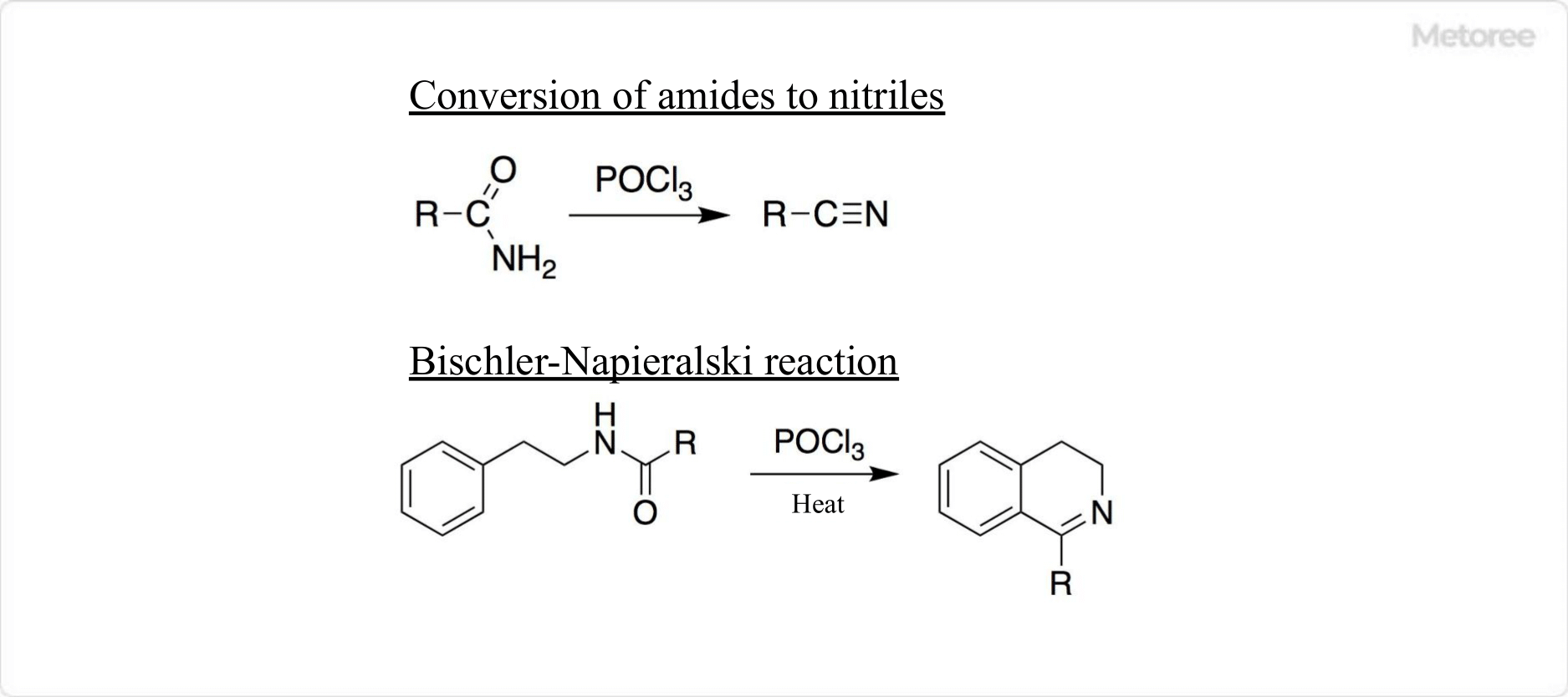
Figure 2. Reaction using phosphorus oxychloride
Phosphorus Oxychloride is often used in the laboratory as a dehydrating reagent. Specifically, it is used in the conversion of amides to nitriles.
Phosphorus Oxychloride can also be used in the Bischler-Napieralski reaction. In other words, the amide precursor undergoes ring closure to produce a dihydroisoquinoline derivative.
3.Birsmeier-Hack Reaction Using Phosphorus Oxychloride
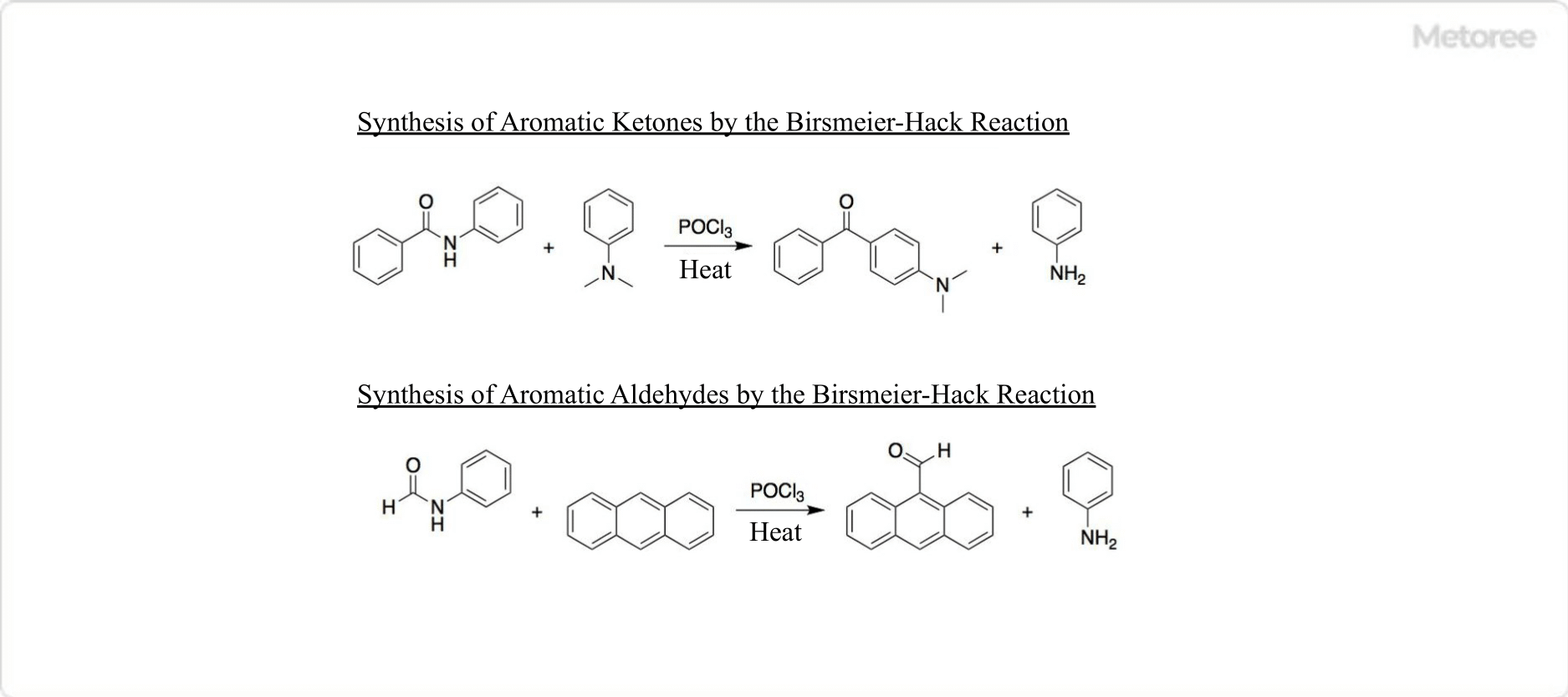
Figure 3. Birsmeier-Hack reaction with phosphorus oxychloride
The Vilsmeier-Haack reaction is a reaction between an active aromatic compound and an amide in the presence of phosphorus oxychloride. Phosphorus oxychloride activates the aromatic ring, which acylates to form aromatic aldehydes and ketones.