What Is a Reverse Osmosis System?

Reverse osmosis membrane water purifiers are water purifiers that use a semi-permeable membrane that allows only water molecules to pass through.
It is also known as a reverse osmosis (RO) water purifier. Semi-permeable membranes have tiny pore sizes (less than 2 nanometers) and can remove substances (minerals, bacteria, viruses, trihalomethane, chlorine, environmental pollutants, heavy metals, radioactive substances, etc.) that cannot be removed by ordinary water filters (hollow fiber membrane, activated carbon, etc.).
Reverse osmosis systems use the phenomenon of reverse osmosis. Reverse osmosis applies pressure to the aqueous solution (raw water) to move water molecules through a semi-permeable membrane to produce pure water that is as close to 100% pure as possible. The resulting pure water does not contain any minerals (calcium, magnesium, inorganic salts, etc.) and is therefore so-called “super” soft water.
Uses of Reverse Osmosis Systems
Reverse osmosis systems were originally developed to desalinate seawater for drinking water, but have since been used for a variety of purposes. These include recycling domestic wastewater into drinking water in outer space, purifying highly pure water for experiments and medical use, ensuring drinking water during disasters, and softening hard water.
Especially in the medical industry, the technology used in reverse osmosis systems is applied as water treatment equipment in dialysis and is an indispensable technology. In 2011, due to the accident at the Fukushima Daiichi Nuclear Power Plant after the earthquake, radioactive materials above the standard values were detected in tap water in the Tokyo metropolitan area, including the Kanamachi Water Treatment Plant.
This led to the focus of attention on reverse osmosis systems, which can remove radioactive substances from tap water.
Principle of Reverse Osmosis Systems
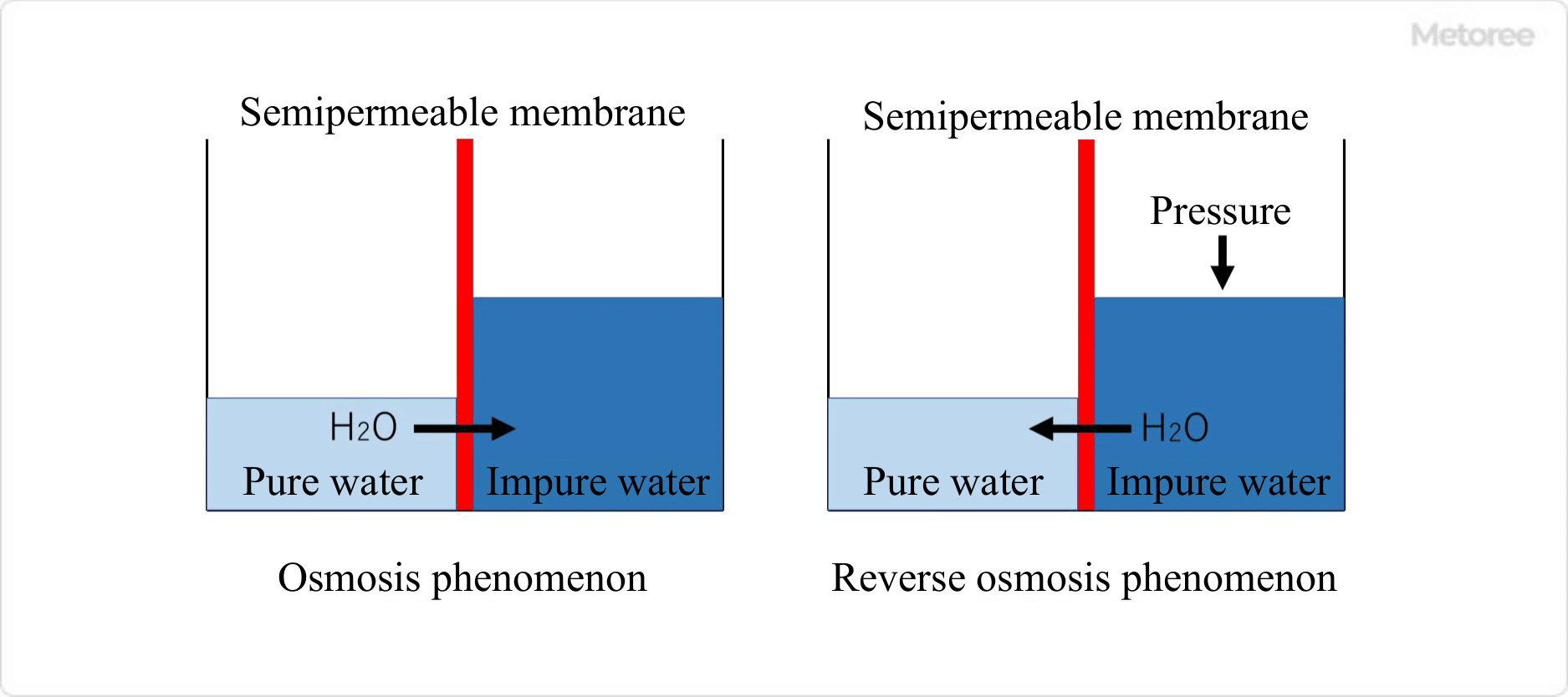
Figure 1. Principle of reverse osmosis membrane water purifiers
Reverse osmosis systems use semi-permeable membranes that allow only molecules and ions below a certain size to pass through. The semipermeable membrane is made of recycled cellulose, acetyl cellulose, or polyacrylonitrile. Osmosis is a phenomenon in which water molecules move from the low-concentration aqueous solution (pure water) to the high-concentration aqueous solution (impure water) through the semipermeable membrane when the high-concentration aqueous solution (impure water) is on one side and the low-concentration aqueous solution (pure water) is on the other side.
Reverse osmosis systems use this principle. Reverse osmosis systems are generally unable to convert raw water containing impurities into 100% pure water, resulting in wastewater that is approximately twice the amount of pure water that can be produced.
In addition, when treating aqueous solutions (raw water) that contain many impurities, such as seawater, pretreatment is necessary according to the concentration and turbidity of impurities in the raw water because semipermeable membranes quickly become clogged if raw water is passed directly through RO modules, which cause reverse osmosis. Furthermore, if there is a high concentration of impurities (ions, etc.) that cannot be removed by pretreatment, it is necessary to increase the pressure applied to the aqueous solution side, which requires the use of a pump to pressurize the solution.
Other Information on Reverse Osmosis Systems
1. Water Treatment Process of Household Reverse Osmosis Systems
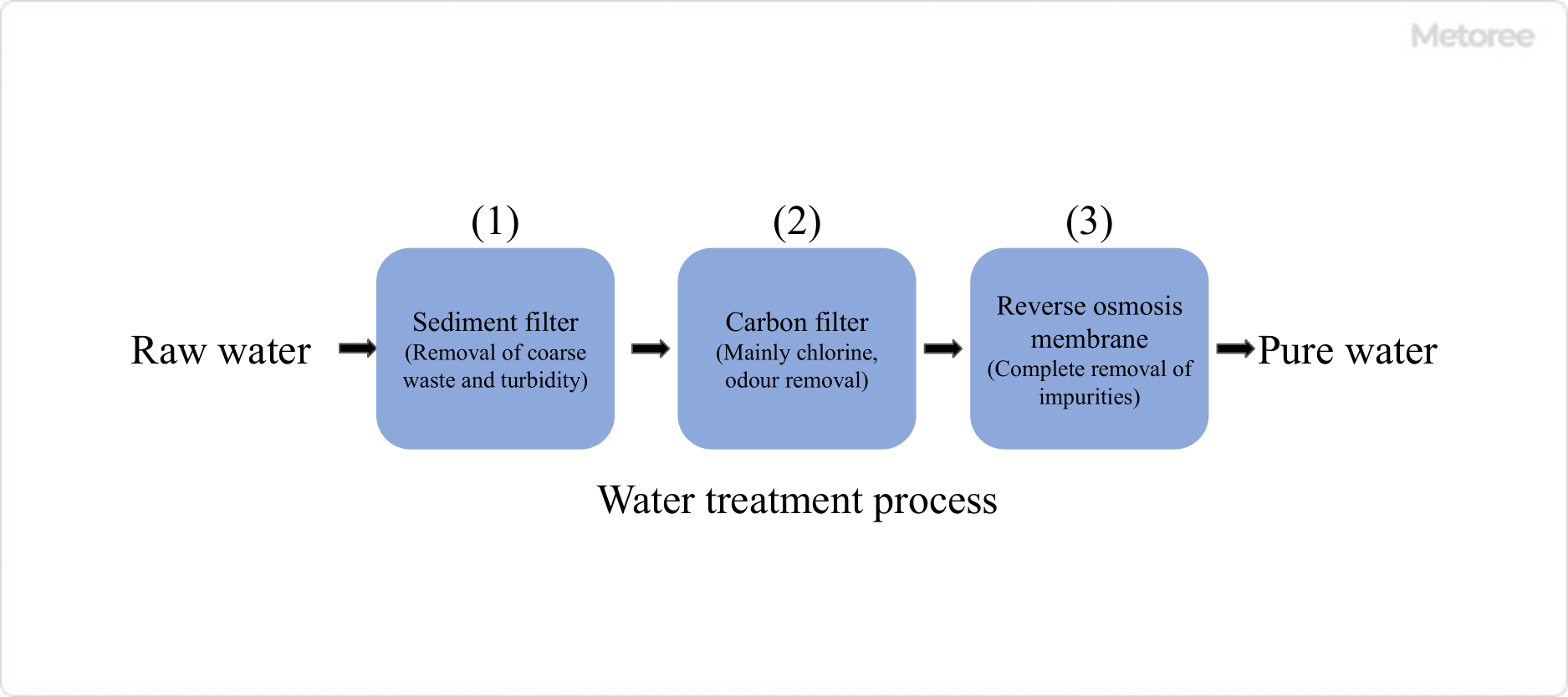
Figure 2. Water treatment processes in household reverse osmosis membrane water purifiers
In the case of household reverse osmosis systems, raw water is first passed through a cement filter. The pore size of the cement filter is usually about 1 to 5 μm. Next, the treated water is passed through an activated carbon (carbon) filter to remove chlorine and odors.
The semipermeable membranes normally used in reverse osmosis are sensitive to chlorine and require chalky (chlorine) removal. The treated water is then passed through a reverse osmosis membrane module to produce pure water through the reverse osmosis phenomenon.
2. Advantages and Disadvantages of Reverse Osmosis Systems
Reverse osmosis systems have the advantage of producing extremely pure water, but there are disadvantages, such as the inability to convert 100% of raw water to pure water (wastewater is generated). There is also the need for pressurization (pump), and the need for appropriate pretreatment depending on the raw water conditions. In addition, reverse osmosis membrane water purification systems have some disadvantages.
In addition, reverse osmosis systems are more expensive than other water purifiers because of their complex system structure due to the principle of reverse osmosis. Therefore, it is necessary to select a purified water production system in consideration of the purpose of use and the level of purity of water required.