What Is Boron Trichloride?
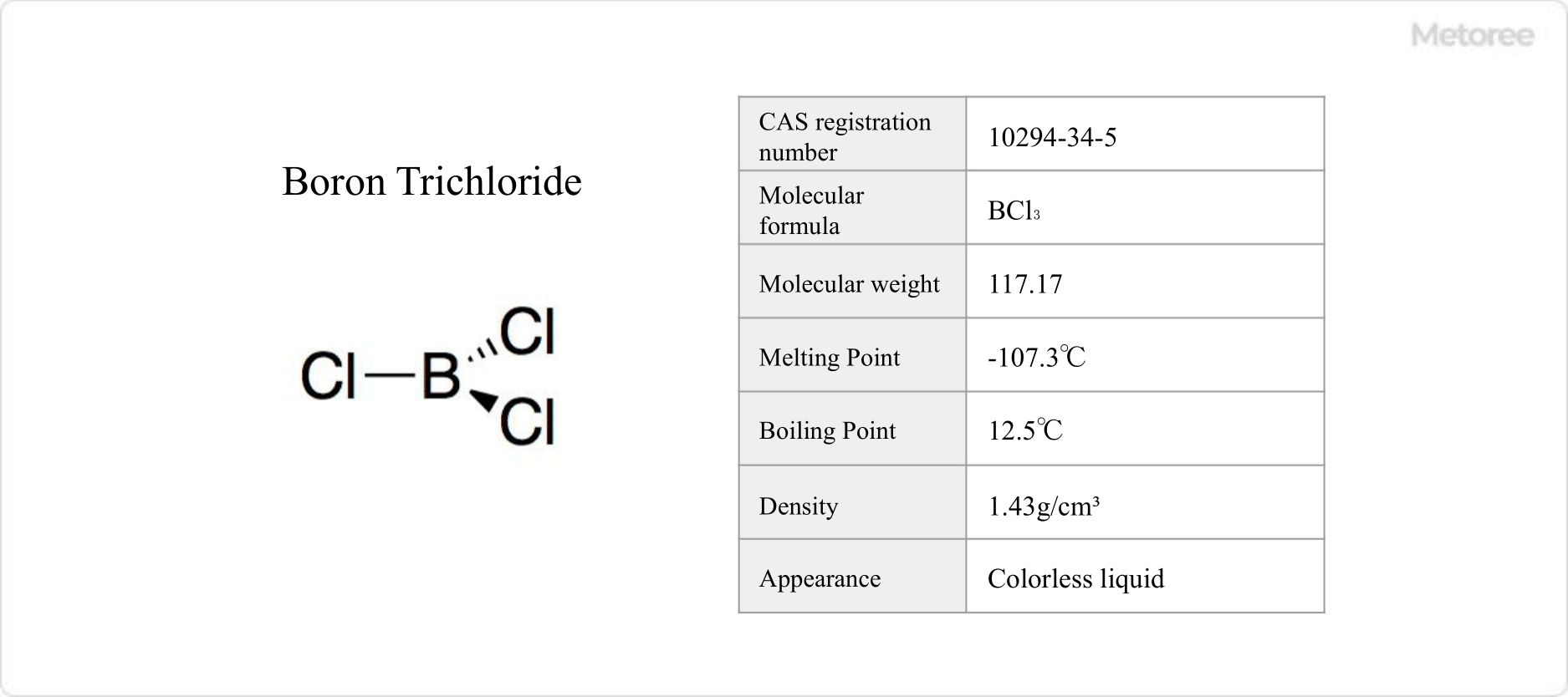
Figure 1. Basic Information on Boron Trichloride
Boron trichloride, consisting of one boron (B) atom bonded to three chlorine (Cl) atoms, is an inorganic compound with the chemical formula BCl3. At standard conditions, it is a colorless, nonflammable, toxic gas with a pungent odor, lacking both a flash and ignition point.
Produced industrially by chlorinating boron oxide at 500°C with carbon, or in the lab via a halogen-exchange reaction between aluminum chloride and boron trifluoride, boron trichloride has vital applications in various sectors.
Uses of Boron Trichloride
Primarily used as a dry-etching gas for fine aluminum wiring in semiconductors and liquid crystal panels, boron trichloride also serves as a raw material in the production of agrochemicals, pharmaceuticals, catalysts, boron nitride (BN), and various chemical vapor deposition (CVD) products.
Properties of Boron Trichloride
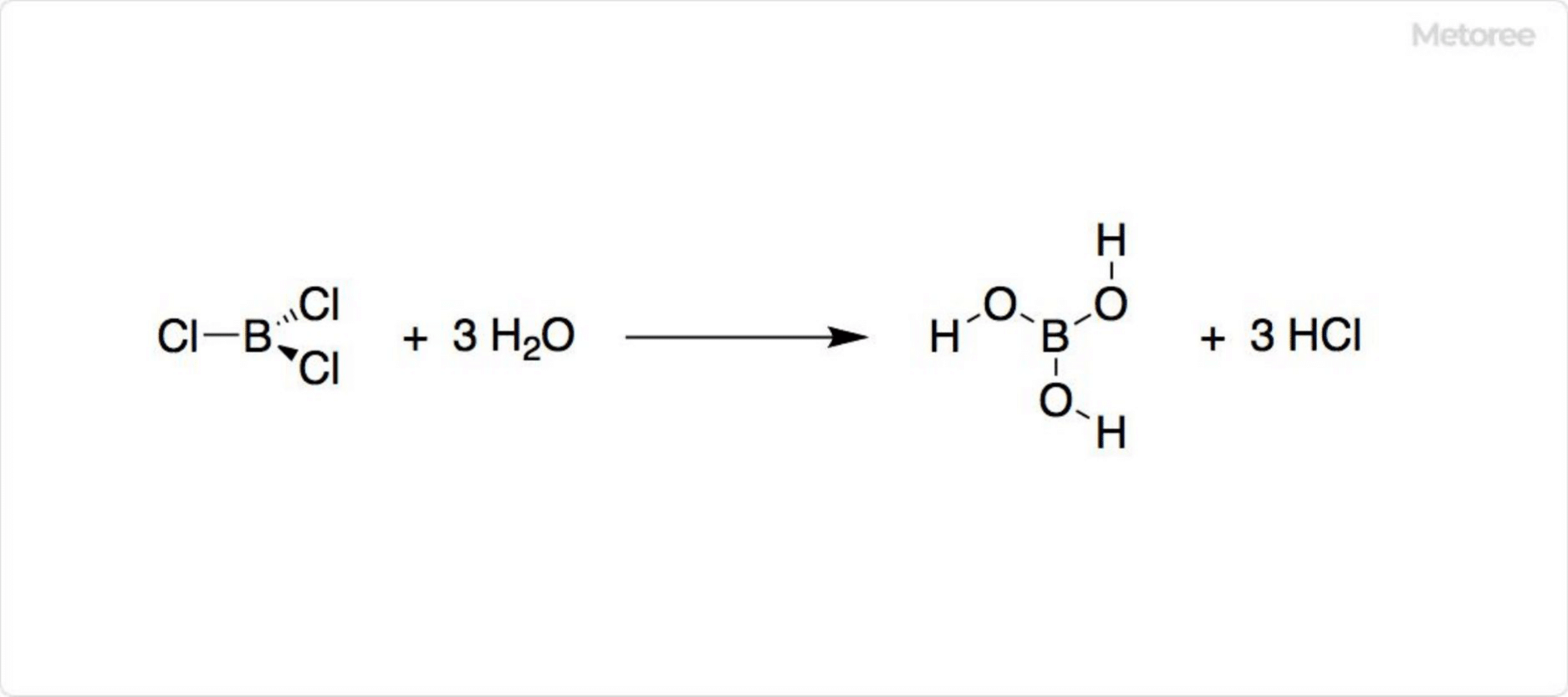
Figure 2. Hydrolysis of Boron Trichloride
With a melting point of -107.3°C and boiling point of 12.5°C, boron trichloride is ether-soluble. Its hydrolysis produces hydrochloric and boric acids, reacting with alcohol to form boric esters. Handling requires caution as moisture and alcohols generate hydrogen chloride. Solid BCl3・S(CH3)2 is a safer, easy-to-handle source, releasing BCl3 upon decomposition by H2O.
Structure of Boron Trichloride
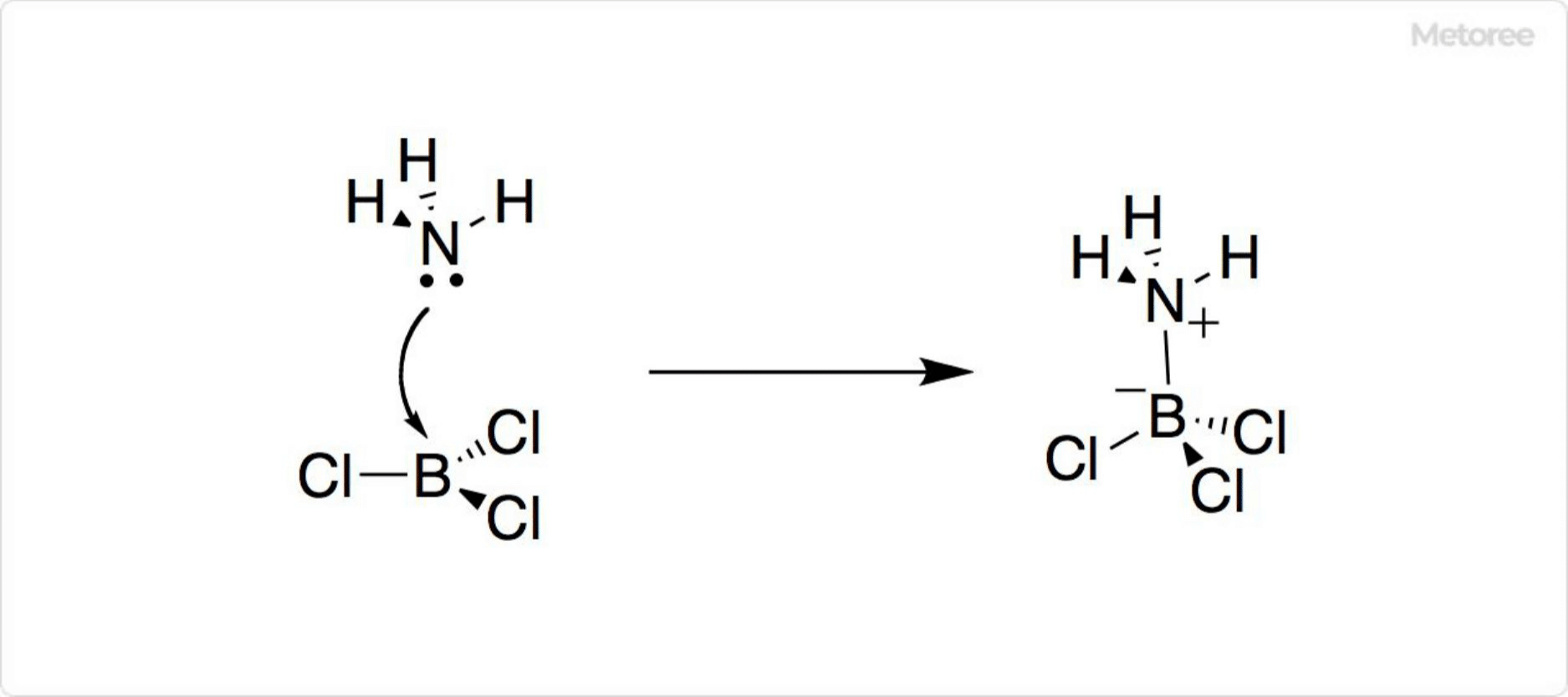
Figure 3. Reaction of Boron Trichloride
A strong Lewis acid, boron trichloride forms adducts with various compounds. Its molecular structure is planar triangular, contributing to its reactivity and formation of complexes.
Other Information on Boron Trichloride
1. Reaction of Boron Trichloride
Oxygen addition produces boron oxide (B2O3), while hydrogen addition yields solid boron. Copper heating generates diboron tetrachloride (B2Cl4), which decomposes at room temperature into compounds with a B cluster structure.
2. Characteristics of Boron Chloride
Diboron tetrachloride, a colorless liquid at room temperature, reacts with water, chlorine, and oxygen to form various compounds. Tetraboron tetrachloride, a pale-yellow crystal, ignites spontaneously in dry air and produces hydrogen by hydrolysis.