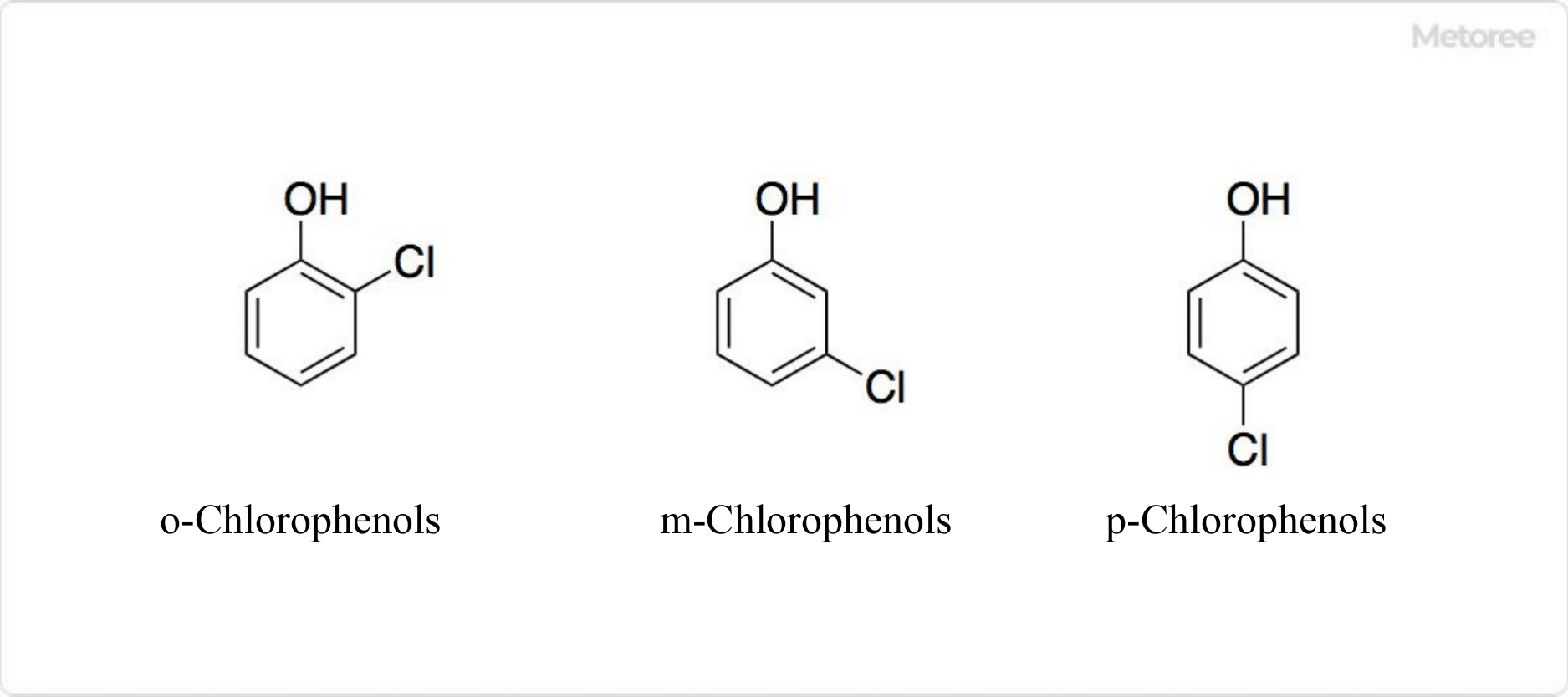
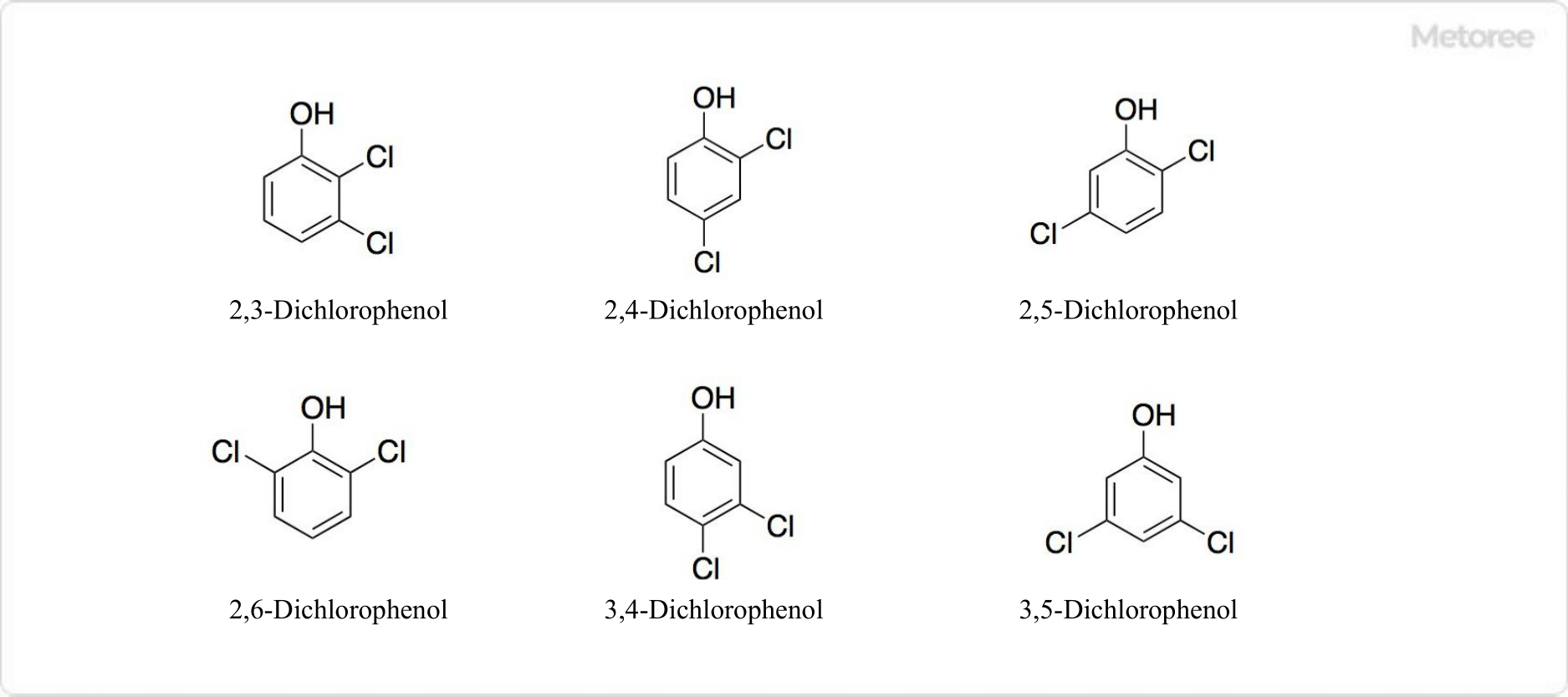
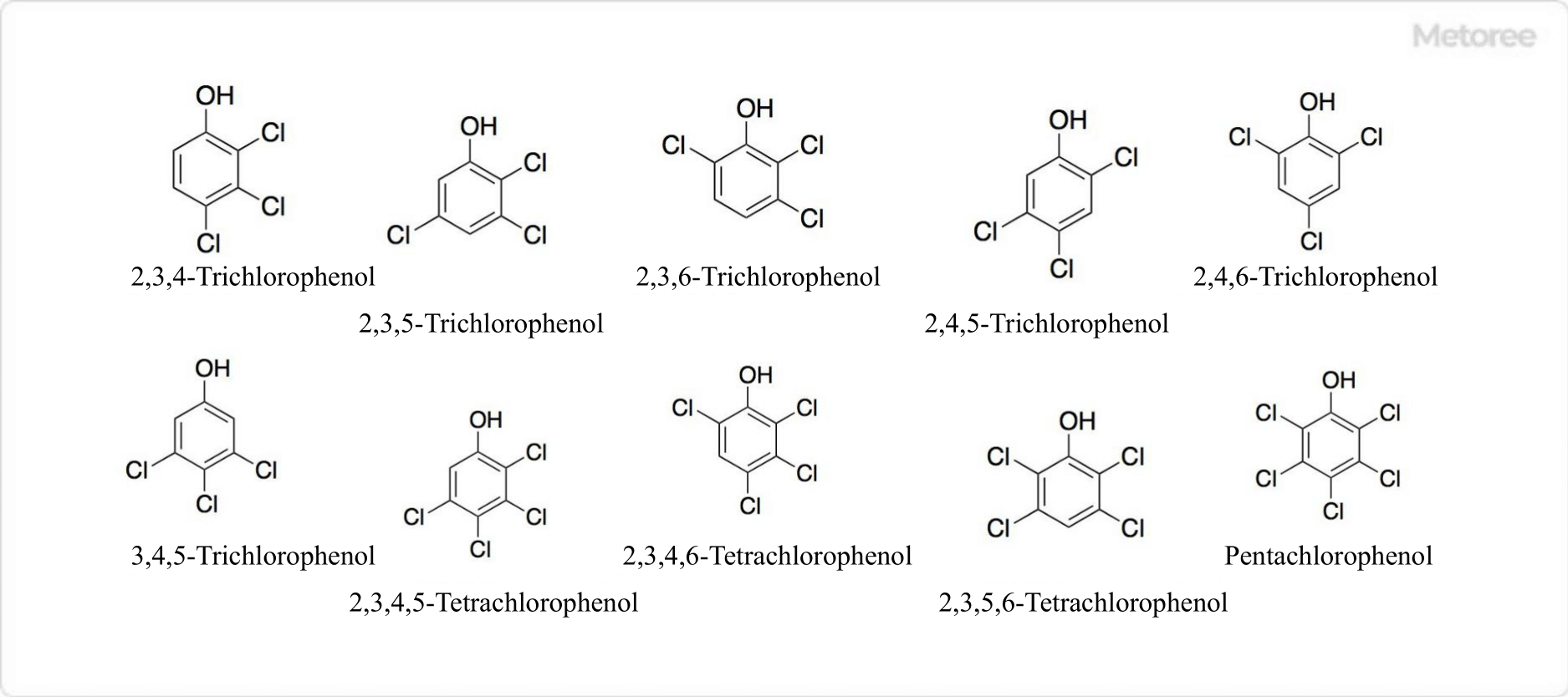
What Is a Chloride?
Chloride refers to chloride, a generic term for compounds in which chlorine forms with a more positive atom or group of atoms.
In nomenclature, compounds with chlorine atoms are denoted either by prefixing “chloride” or “chloro” or by suffixing “chloride. Chlorine Cl2 can react with almost any element except Group 18 elements to form chlorides, and if the chlorine bond is ionic, it readily liberates the chloride ion Cl−.
Inorganic chlorides containing chlorine atoms are in most cases ionically bonded. In water, they ionize into chloride ions and inorganic cations and readily dissolve in water.
Uses of Chlorides
Chlorides (chlorides), which are compounds of chlorine, are used throughout our daily lives.
1. Hydrogen Chloride
Hydrogen chloride is a substance with the molecular formula HCl. It is a gas at room temperature, soluble in water, and its aqueous solution is highly acidic. Taking advantage of this feature, hydrochloric acid is used not only as a catalyst and neutralizer in many chemical reactions, but also in acid catalytic reactions, neutralization of alkaline substances, and in the formation process of sodium hydroxide (caustic soda). In addition, it is sometimes used industrially to clean and remove corrosion from metal surfaces.
2. Oxoacid
Oxoacids are acids that contain oxygen atoms in their molecules. There are four types: hypochlorous acid HClO, chlorous acid HClO2, chloric acid HClO3, and perchloric acid HClO4. Aqueous solutions of HClO hypochlorite have bleaching and disinfecting properties and are used to bleach clothes and disinfect tap water. Of these salts, potassium chlorate KClO3 has strong oxidizing properties. It is therefore used as an oxidizing agent in matches and gunpowder.
3. Halogen Salts
Halogen salts are salts in which halogens are compounded with metallic elements such as sodium and potassium. Most of them are easily soluble in water, but silver halide and lead(II) halide, other than fluorine, are insoluble in water.
Silver halide is photosensitive to light, absorbing light energy when exposed to light and changing into silver ions and halide ions. This is the mechanism by which dark and light areas are formed on photographic film and photosensitive paper.
4. Organic Chlorides
Organic chlorides containing chlorine atoms are stable covalent compounds in which the chlorine atom is bonded to the sp3 carbon. chloro groups substituted on the sp3 and sp2 carbons are easily desorbed, and such organic chlorides are used as substrates for desorption reactions. In nucleophilic substitution reactions such as Williamson synthesis, the chloro group on the sp3 carbon is also used as a leaving group.
For example, benzyl chloride (benzyl chloride) is used in organic synthesis to replace the hydrogen of the OH group of alcohols, carboxylic acids, and phenols with a benzyl group. This is one method of protecting the OH group with a benzyl group (benzyl protection).
Properties of Chlorides
Chlorides are based on the properties of chlorine alone and its compound, Chloride.
1. Properties of Chlorine
Chlorine, also called halogen, is a group of 17 elements on the periodic table. The word halogen comes from the Greek words halos (salt) and Gennaro (to make).
It has a valence electron number of 7 and a high electron affinity, making it easy to form a monovalent anion. In nature, it does not exist by itself but exists as a compound.
| Molecular Formula |
Cl2 |
| Stand-alone state (At Room Temperature and Pressure) |
Yellow-green gas |
| Molecular Weight |
71 |
| Boiling Point (°C) |
−101 |
| Oxidizing Power |
Strong |
Chlorine is highly oxidizing, so when dissolved in water, some of it reacts to form hydrogen chloride HCl and hypochlorous acid HClO. These substances are typical Chlorides.
2. Properties of Chlorides
Chlorides are compounds with a single chlorine atom as the anion. The chloride ion is a monovalent anion without charge. They generally combine with metals to form ionic crystals, but some compounds contain carbon and hydrogen, such as organic chlorides.
Chlorides occur in many forms in nature and are well known to be a major component of salt in seawater. Sodium chloride (salt) is another example of chloride. Chlorides play an important role in many chemical reactions and industrial processes.
For example, hydrogen chloride (hydrochloric acid) is widely used as a strong acid, and alkyl chloride is used in organic synthesis. In addition, iron chloride is useful as a rust inhibitor and pigment.
Other Information on Chlorides
Utilization of Chlorides
Some chlorides are used as ingredients in shampoo and treatment products. Ingredients in shampoo formulations include detergents, surfactants, water, and fragrance. Some chlorides are included as surfactants.
Because our hair is negatively charged, chlorides used as surfactants are strongly adsorbed and highly persistent. Some of the most commonly used are behentrimonium chloride, steartrimonium chloride, and distearyldimonium chloride.
Recent studies have shown that these substances have a negative effect on hair quality because they have a strong protein denaturing effect and are difficult to wash out.