What Is a Liquid Nitrogen Container?
 A liquid nitrogen container is a container used for storing, preserving, and transporting liquid nitrogen. Liquid nitrogen is nitrogen in liquid form at temperatures below -196°C and is mainly used to cool objects.
A liquid nitrogen container is a container used for storing, preserving, and transporting liquid nitrogen. Liquid nitrogen is nitrogen in liquid form at temperatures below -196°C and is mainly used to cool objects.
If used incorrectly, it can cause explosions, asphyxiation, frostbite, and other serious accidents, so it must be handled with care. By using liquid nitrogen storage containers, it is possible to safely and efficiently store, preserve, and transport liquid nitrogen.
Uses of Liquid Nitrogen Containers
Liquid nitrogen containers are used for storage and transportation when liquid nitrogen is used in laboratories and medical institutions where samples are stored and experiments are conducted, in food-related flash freezing, and as a coolant for IT-related equipment. Liquid nitrogen is also used in special analysis equipment.
There are two types of liquid nitrogen containers: open containers and sealed containers. The most common open containers are small to medium-sized containers, while the most commonly sealed containers are large containers with a large capacity. An open container is one in which a cap is simply placed over the mouth of the liquid nitrogen containers and is not fixed, while a closed container is one in which the cap is fixed and the container is hermetically sealed.
Liquid nitrogen containers are not suitable for long-term storage, but they are suitable for daily or short-term use. Liquid nitrogen containers can be used for short-distance transportation, short-term storage and preservation, and periodic replenishment.
Principle of Liquid Nitrogen Containers
Nitrogen expands approximately 700 times in volume when it changes from a liquid to a gas, leading to accidents such as explosions and lack of oxygen. Therefore, liquid nitrogen containers are insulated with a double-layered vacuum structure to stabilize the temperature with high thermal insulation.
In addition, the structure is resistant to shock and vibration to withstand breakage. However, even when stored in liquid nitrogen containers, it will vaporize little by little.
Structure of Liquid Nitrogen Containers
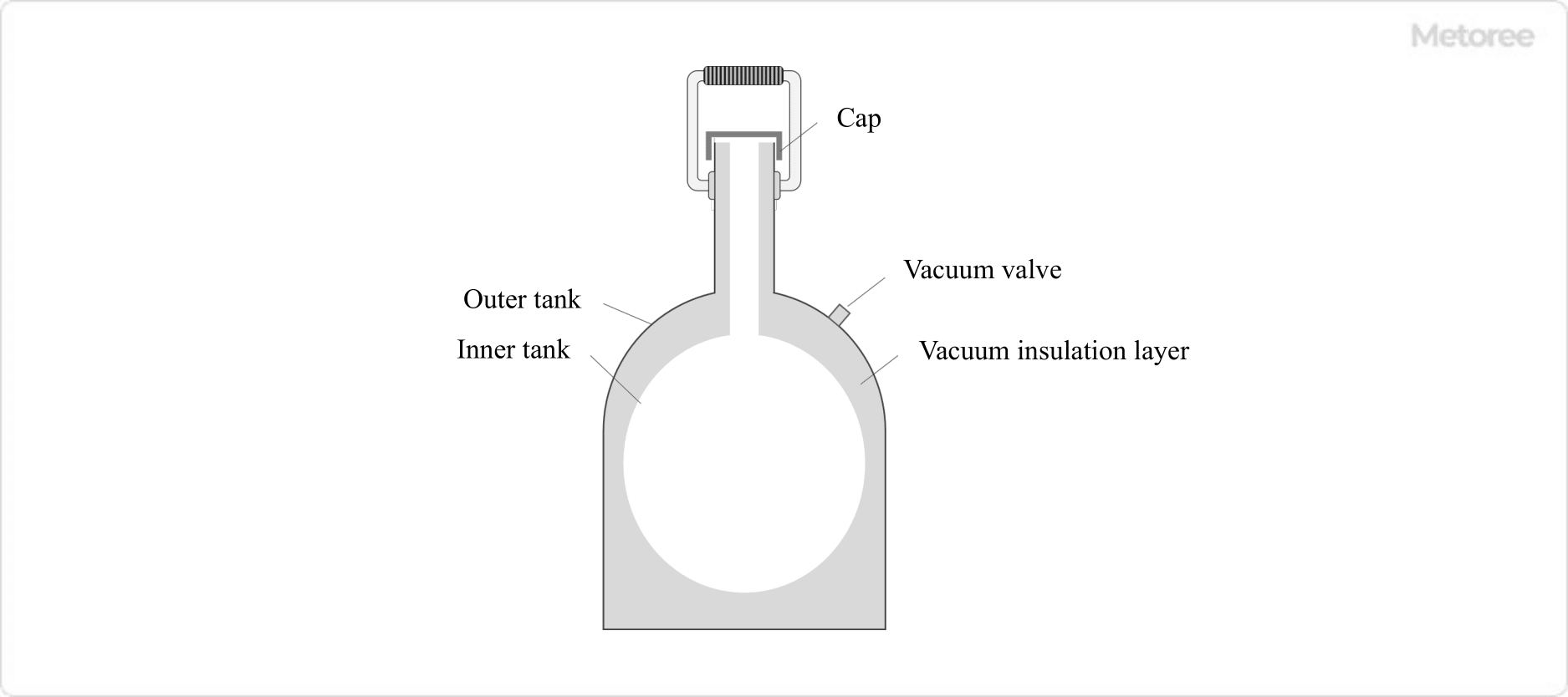
Liquid nitrogen containers consist of an outer tank that forms the outside of the container an inner tank that is filled with liquid nitrogen, and a vacuum insulation layer is placed between the outer and inner tanks. Open-type liquid nitrogen containers have a simple structure that is not fixed by simply placing a cap over the open end of the inner tank and is widely used in university laboratories.
In the open type, the outer tank is generally made of aluminum, and the inner tank is generally made of hard glass, metal, or FRP (fiberglass reinforced plastic).
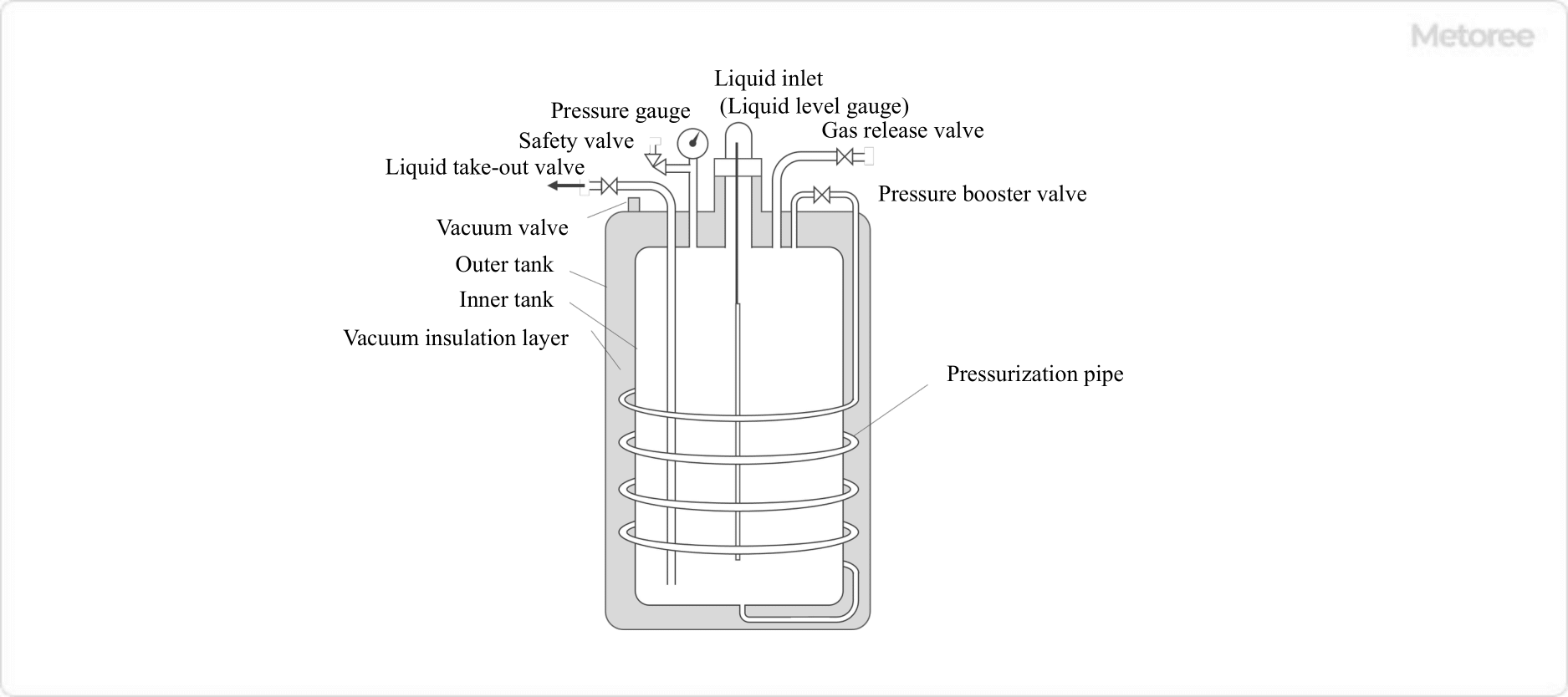
A self-pressurizing (sealed) liquid nitrogen container has a structure in which a pressurizing coil is placed between the outer and inner tanks, and liquid nitrogen is extracted by increasing the pressure of the inner tank to pressurize the liquid nitrogen in the inner tank.
The self-pressurizing type has a liquid takeout valve placed on the liquid nitrogen takeout side, a pressure boosting source valve used to increase the pressure of the inner tank, and a gas release valve to control an excessive increase in the internal pressure in the inner tank, and by adjusting these control valves, the required amount of liquid nitrogen can be taken out.
Other Information on Liquid Nitrogen Containers
1. How to Use Liquid Nitrogen Containers
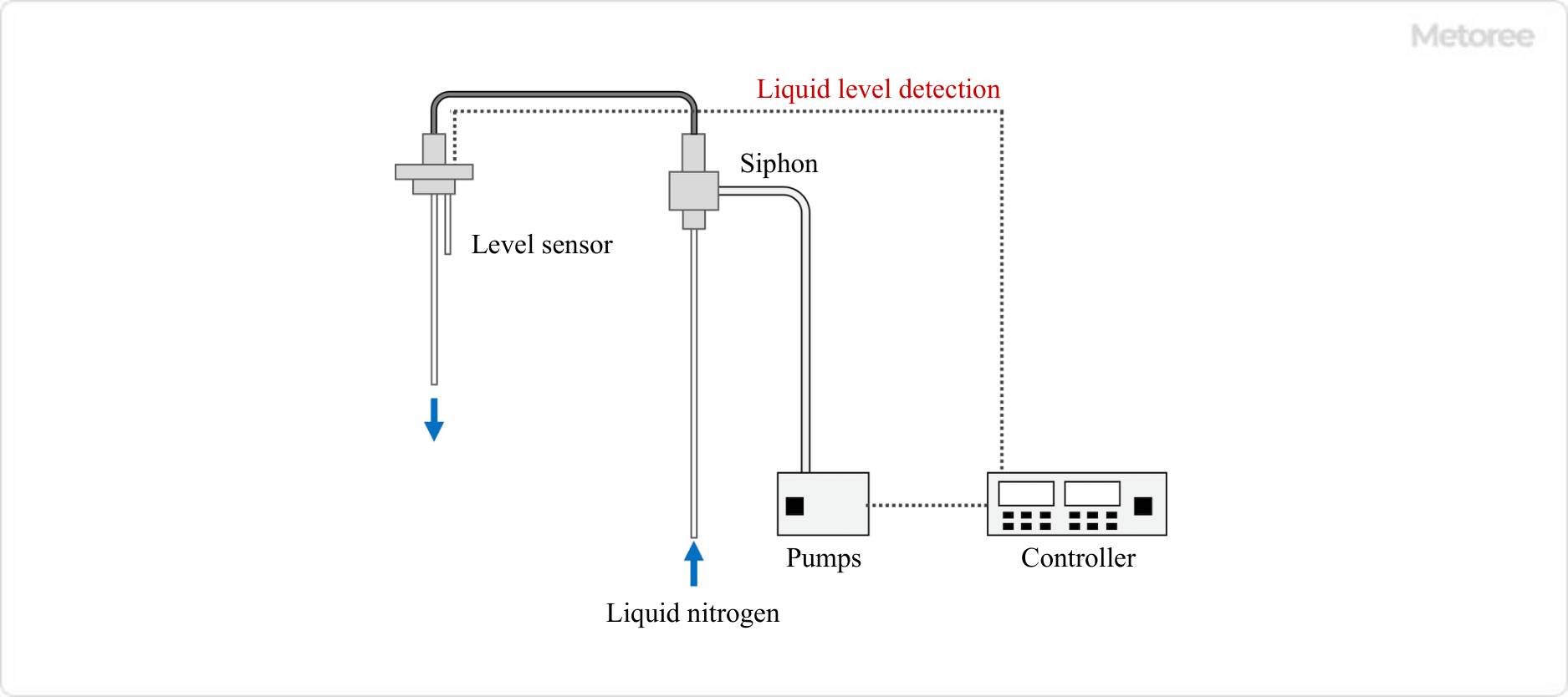
Since open-type liquid nitrogen containers are small and relatively lightweight, liquid nitrogen can be taken out by lifting and tilting the container. However, for safety reasons, it is recommended that a siphon or similar device be used to remove the liquid nitrogen.
There are two types of siphons: a manual type using a rubber ball and a type that automatically pumps liquid using a controller. In particular, since it is difficult to see the liquid level inside a dewar for cooling the EDX detector of an electron microscope, a siphon with a level sensor can prevent liquid nitrogen from overflowing and cover precision instruments with liquid nitrogen.
When storing containers, special lids must be used to prevent water from freezing inside and blocking extraction. In the unlikely event that the lid is lost or otherwise inaccessible, the container should be covered with a dry rag to prevent it from opening to the atmosphere while avoiding hermetic sealing.
The inner tank has only the minimum necessary structure and is fixed in place. For this reason, it is vulnerable to lateral forces and requires careful storage. Self-pressurized (sealed) liquid nitrogen containers have a structure in which gas is extracted by adjusting multiple valves, and care must be taken in handling each adjusting valve.
To remove liquid from the container, start by closing the gas release valve and opening the pressurization valve and then confirm the internal pressure of the container is rising. Then, open the liquid takeout valve and take out the liquid nitrogen containers while making sure that the pressure inside the container does not rise excessively.
After the liquid nitrogen has been taken out, close the liquid nitrogen withdrawal valve and the pressurizing valve in turn, and then open the gas release valve to lower the internal pressure of the container.
Precautions When Using Liquid Nitrogen Containers
When handling liquid nitrogen, care must be taken to avoid asphyxiation, frostbite, and explosions. To prevent frostbite, use gloves and face guards during use, and wear footwear that is impervious to liquid nitrogen, since slippers and the like may come into direct contact with liquid nitrogen when it is spilled.
Similarly, it is recommended to use dry, specialized gloves, as military gloves and the like may be penetrated. If liquid nitrogen is to be moved in and out indoors, the room must be well ventilated. Also, when using an elevator, do not share the elevator with liquid nitrogen because the interior is a closed room.
If liquid nitrogen is placed in an elevator, put up a sign or other warning so that others will not get on the elevator. For rooms where liquid nitrogen is frequently used, we recommend installing oxygen monitors.
If it is an open type, of course, it is not designed to be sealed, so sealing is strictly prohibited. It is also strictly prohibited to leave the equipment without a lid, as explained in the instructions for use. This is not only because liquid nitrogen vaporizes faster, but also because water and oxygen may liquefy and mix inside the storage container.
Liquid oxygen, in particular, can react rapidly when it comes into contact with organic matter (liquid oxygen has a bluish tint). Ensure to cover the container with a dry cloth or other covering if the lid is misplaced. The open type is structurally vulnerable to shaking and shock and should be used with care, as the vacuum insulation layer may break.
In the self-pressurizing type, a safety valve maintains the internal pressure in a safe condition, so it should be checked regularly to ensure that the safety valve is functioning properly. It is also dangerous to look into the outlet as it may not be taken out correctly or may blow out suddenly if freezing occurs in the piping.