What Is Sulfurous Acid?
Sulfurous acid, with the chemical formula H2SO3, is believed to exist in aqueous solutions of sulfur dioxide. It belongs to the oxoacids of sulfur, having a CAS number of 7782-99-2. Although the free acid is unstable and cannot be isolated, it is often referred to in solutions where the sulfite ion HSO3– predominates.
Sulfurous acid is commonly found in its aqueous form, and sulfur dioxide gas is sometimes referred to as sulfurous acid gas.
Uses of Sulfurous Acid
Sulfurous acid and sulfur dioxide serve as reducing and bleaching agents. They find applications as food additives, preservatives in dried fruits and liquors, antioxidants, and bleaching agents. Additionally, their uses extend to disinfectants in food processing, agricultural fumigants, insecticides, antiseptics, and preservatives for fruits and vegetables. They are also employed as bleaching agents in the paper, textiles, straw, gelatin, glue, and sugar industries.
Properties of Sulfurous Acid
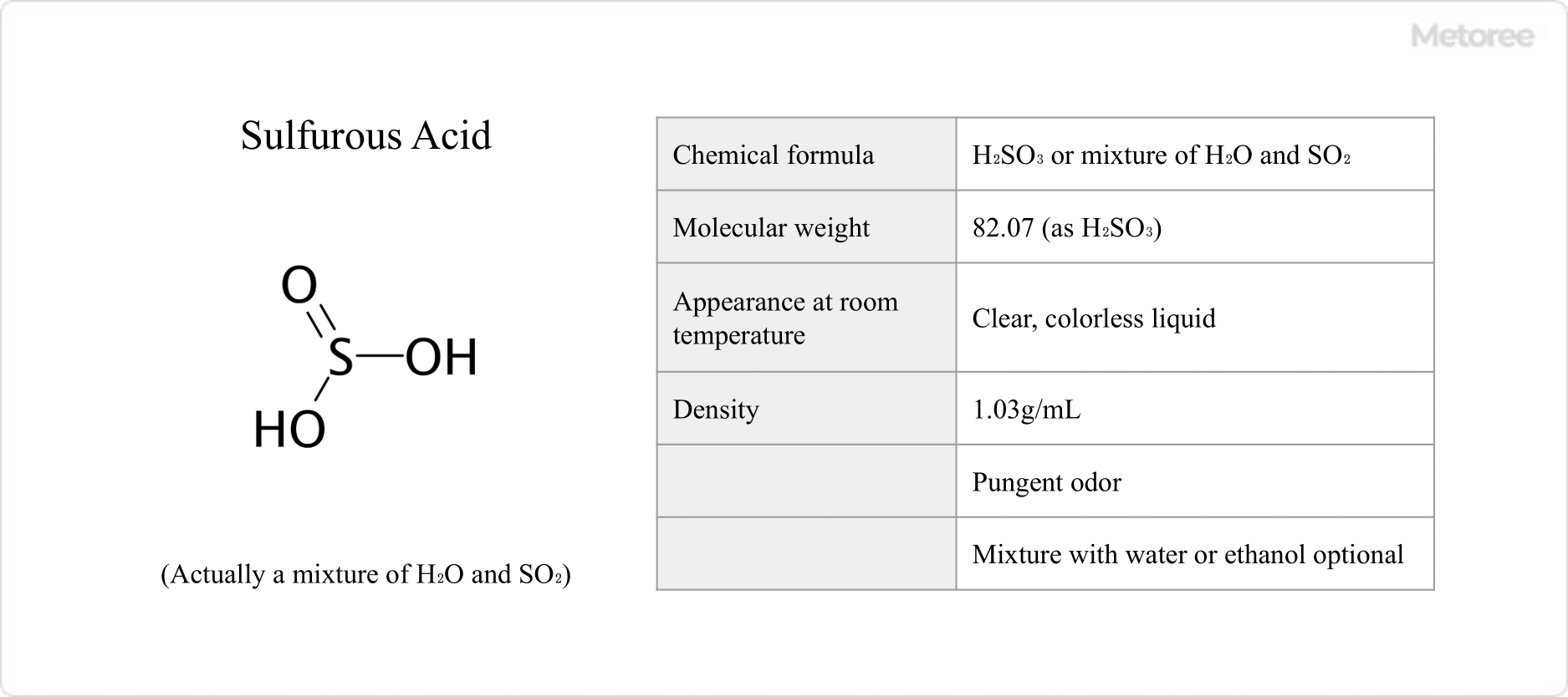
Figure 1. Basic Information on Sulfurous Acid
Sulfurous acid, when mixed with water, creates a clear, colorless liquid with a density of 1.03 g/mL. It has a pungent odor and is miscible with water and ethanol.
Types of Sulfurous Acid
1. Aqueous Sulfurous Acid
Aqueous sulfurous acid is available as a reagent for research and development, typically used as a general analysis reagent and reducing agent. It is available in various volumes, with sulfur dioxide concentrations usually around 5% or 6%. Due to its strong odor, it should be handled in well-ventilated areas.
2. Sulfurous Acid Gas
Sulfur dioxide gas, referred to as sulfurous acid gas, is used as a bleaching and reducing agent in metal production and chemical manufacturing. It is typically stored under high pressure as a liquefied gas and requires careful handling.
Other Information on Sulfurous Acid
1. Equilibrium of Aqueous Sulfurous Acid
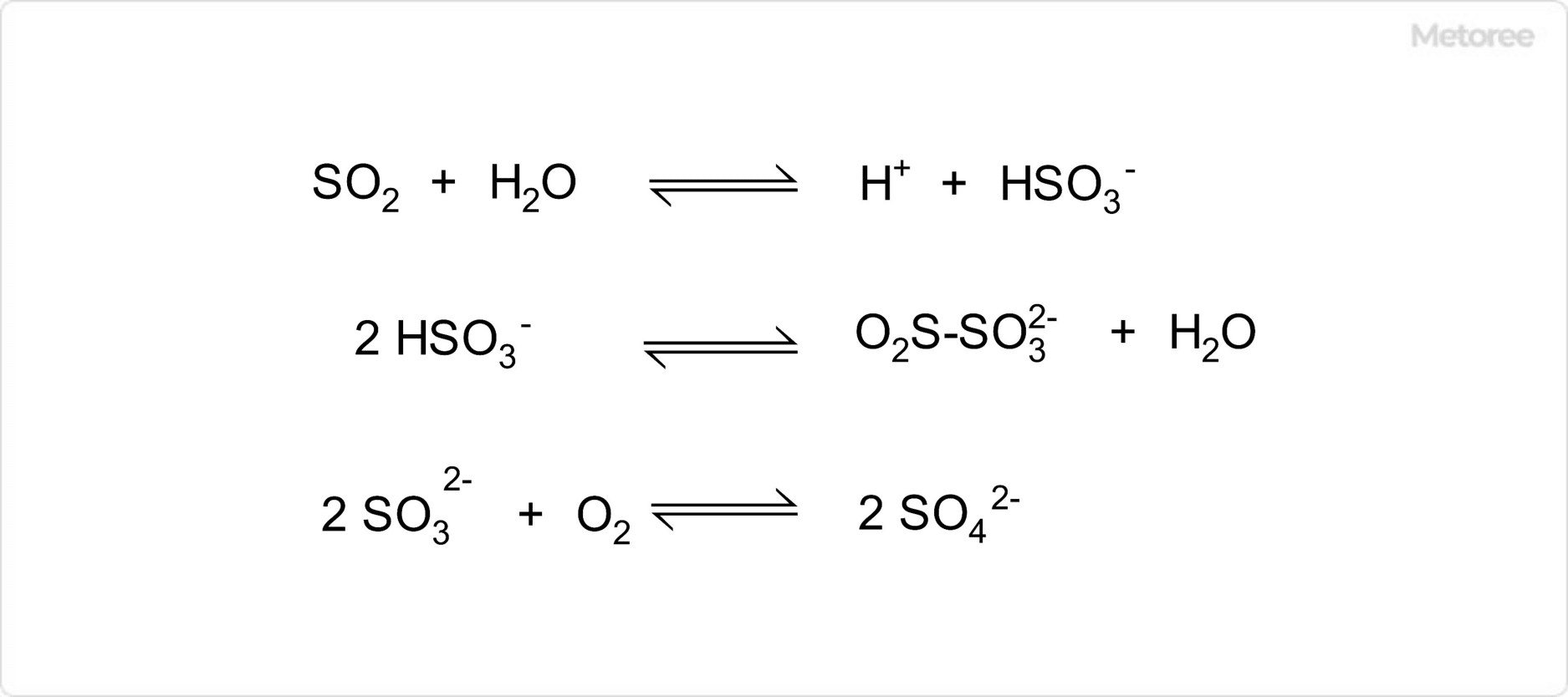
Figure 2. Equilibrium of Aqueous Sulfurous Acid
In aqueous solutions, sulfurous acid is believed to be in equilibrium with its ions, transitioning between hydrogen sulfurous acid ions and their dimerized structures, and further reacting with oxygen to form sulfate ions.
2. Physical Hazards of Sulfurous Acid
Sulfurous acid and sulfur dioxide can be hazardous; heating can increase pressure, posing a rupture risk. They react violently with various substances, including ammonia and chlorine, and can corrode materials upon contact with water or steam.
3. Human Health Hazards of Sulfurous Acid
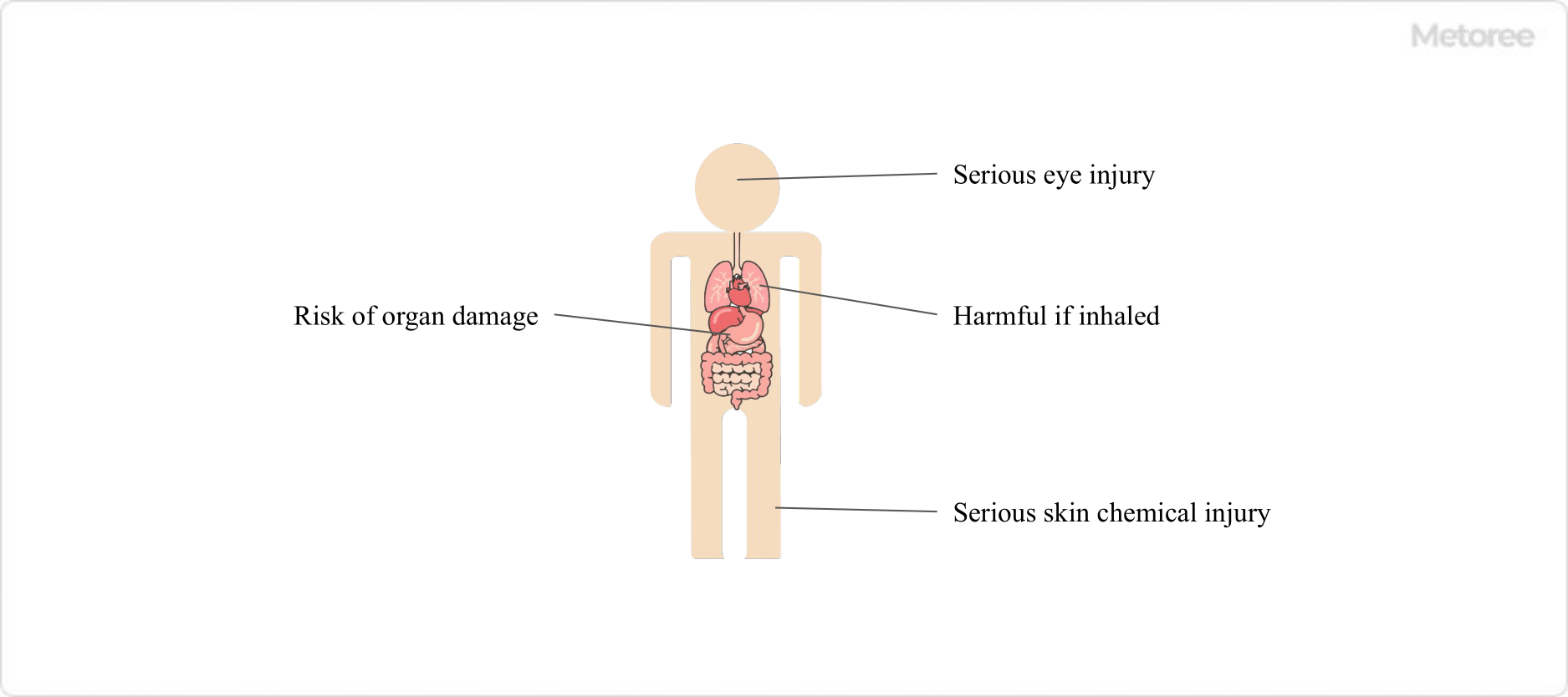
Figure 3. Human Health Hazards of Sulfurous Acid
Sulfurous acid poses several health risks, including serious skin burns, eye damage, inhalation hazards, and potential organ damage with prolonged exposure. Its GHS classification includes acute toxicity, skin corrosion, and specific target organ toxicity.
4. Regulatory Information on Sulfurous Acid
Due to its hazardous nature, sulfurous acid is regulated under various safety laws. Proper handling in compliance with regulatory requirements is essential.