What Is Amyl Acetate?
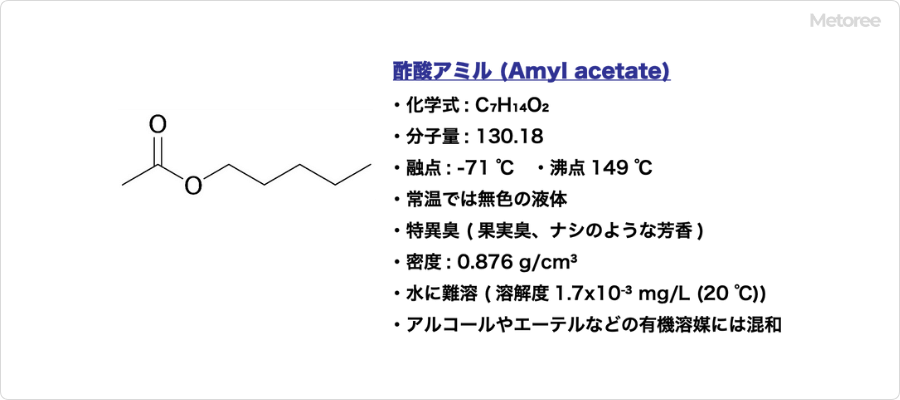
Amyl acetate is an organic compound, the ester of amyl acetate and amyl alcohol.
It has the chemical formula C7H14O2 and molecular formula CH3COO(CH2)4CH3. Other names include pentyl acetate, n-amyl acetate, and n-amyl acetate ester.
It has a molecular weight of 130.18, a melting point of -71°C, and a boiling point of 149°C. Its density is 0.876 g/cm³. It is insoluble in water (solubility 1.7×10-3 mg/L (20°C)), but miscible in organic solvents such as alcohol and ether.
The ester produced from one of the isomers of 1-pentanol or a mixture of these isomers can also be referred to as amyl acetate.
Uses of Amyl Acetate
Amyl acetate is mainly used as solvents and lacquers. These include solvents for perfumes and textile dyes, adhesive manufacturing, oil paints, lacquers, and nitrocellulose solvents.
Other applications include film preparations, such as photographic film and celluloid, a synthetic plastic material.
It is used industrially in various applications.
Pearl essence is a liquid that is sprayed on a round glass bead from the outer layer or hollow interior when making imitation pearls, and amyl acetate is sometimes used in this type of paint solution. It may also be included as an ingredient in stain removers.
Chemistry and Synthesis of Amyl Acetate
1. Synthesis of Amyl Acetate
Amyl acetate can be synthesized by the usual ester synthesis reaction using a carboxylic acid (acetic acid), an alcohol (1-pentanol (n-amyl alcohol)), and an acid catalyst such as concentrated sulfuric acid.
As with other esters in general, it is also possible to synthesize amyl acetate using acid anhydrides or acid halides.
2. Chemical Properties of Amyl Acetate
Amyl acetate undergoes hydrolysis in the presence of an acid or base and is decomposed into acetic acid and 1-pentanol. In reactions using reducing agents such as lithium aluminum hydride (LiAlH4), sodium borohydride (NaBH4), and borane (BH3), alcohols (ethanol and 1-pentanol) are formed.
They are relatively stable to heat and light. However, mixing with nitrates, strong oxidizing agents, strong bases, and strong acids should be avoided. Mixing with these substances may cause fire or an explosion hazard.
The flash point is low at 25°C and the substance should be handled as a flammable liquid or vapor. Combustion produces carbon monoxide and carbon dioxide. There is a risk of mild skin irritation, eye irritation, and respiratory tract irritation in humans, and is slightly toxic.
Types of Amyl Acetate
There are several types of amyl acetate supplied to the market, including common chemical reagent products and industrial chemicals. As a chemical reagent, the most common volumes are 25mL and 500mL, which are easy to handle in the laboratory. It is usually served in a glass bottle. This reagent can be transported and stored at room temperature.
As an industrial chemical, it is traded as a type of solvent and is often handled in large packages such as 15kg oil cans, 180kg drums, and 1,000L containers.