What Is a Nuclear Magnetic Resonance (NMR) Analyzer?
Nuclear magnetic resonance (NMR) analyzers determine the chemical environment of atoms, identifying elements in the surrounding environment and bonding states to elucidate the structure of the analyzed compound.
The results from NMR analyzers are depicted with the chemical shift on the horizontal axis—representing the frequency difference between the NMR signal of the reference substance and the analyzed substance—and intensity on the vertical axis.
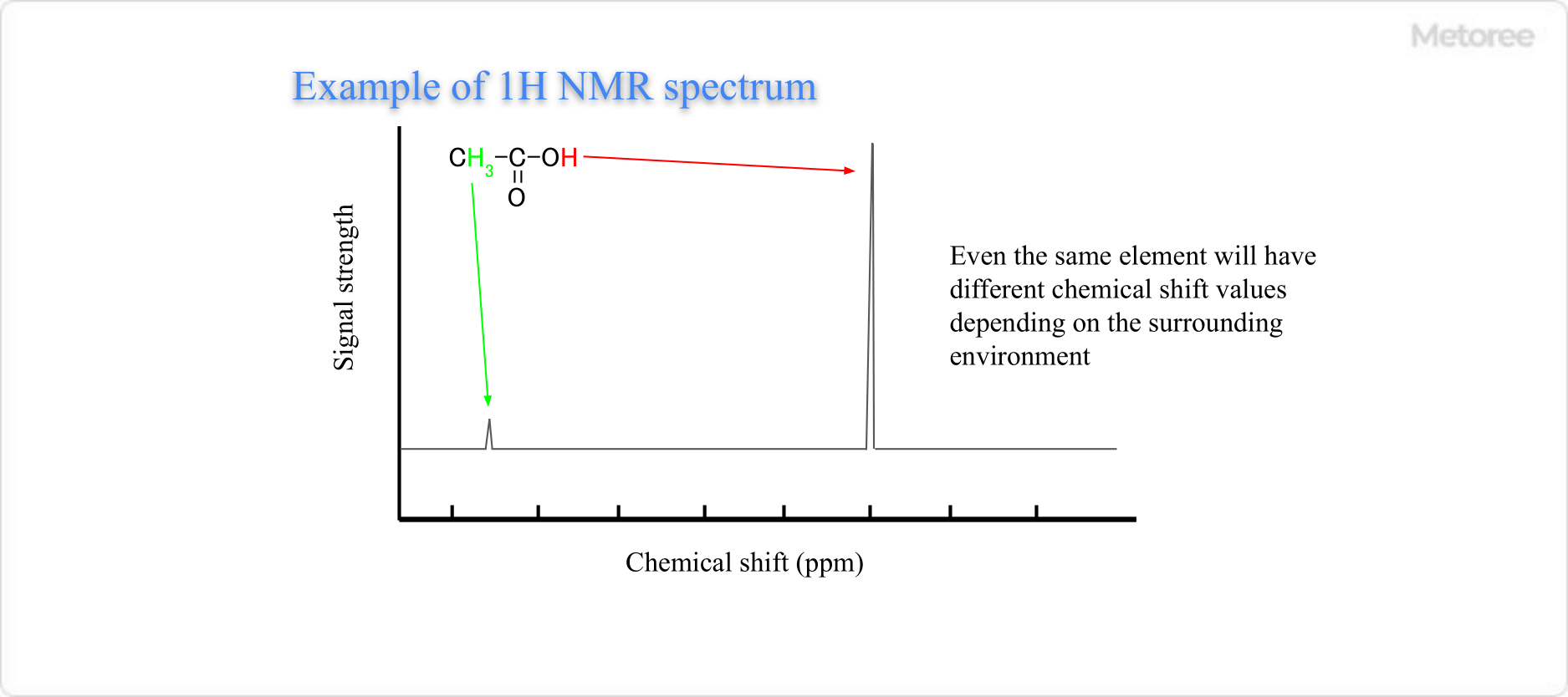
Figure 1. Example of NMR Measurement Results
NMR analyzers, capable of evaluating specific elemental species during measurement, offer detailed insights for each element, even in complex compounds. They accommodate a diverse array of samples, including liquids, solids, and gel-like substances.
While Raman spectrophotometers and electron microscopes also facilitate structural analysis, NMR analyzers provide simple, nondestructive analysis, offering comprehensive structural information, including data on neighboring atomic species.
Applications of NMR Analyzers
NMR analyzers find applications beyond materials analysis, extending into clinical fields, as highlighted below.
1. Material Analysis
NMR excels in analyzing organic materials, including resins, biomaterials, and electrolytes for batteries, aiding in structural elucidation and degradation cause analysis.
It clarifies structures of synthesized or purified substances, identifies molecular weight components, assesses purity, detects impurities, and facilitates quantitative analysis through database comparisons.
2. Clinical Applications
MRI (magnetic resonance imaging), utilizing NMR principles, visualizes the body’s water distribution, offering detailed tissue state images without radiation exposure risks, surpassing CT scans in resolution and detection capabilities.
Principle of NMR Analyzers
1. Nuclear Magnetic Moment
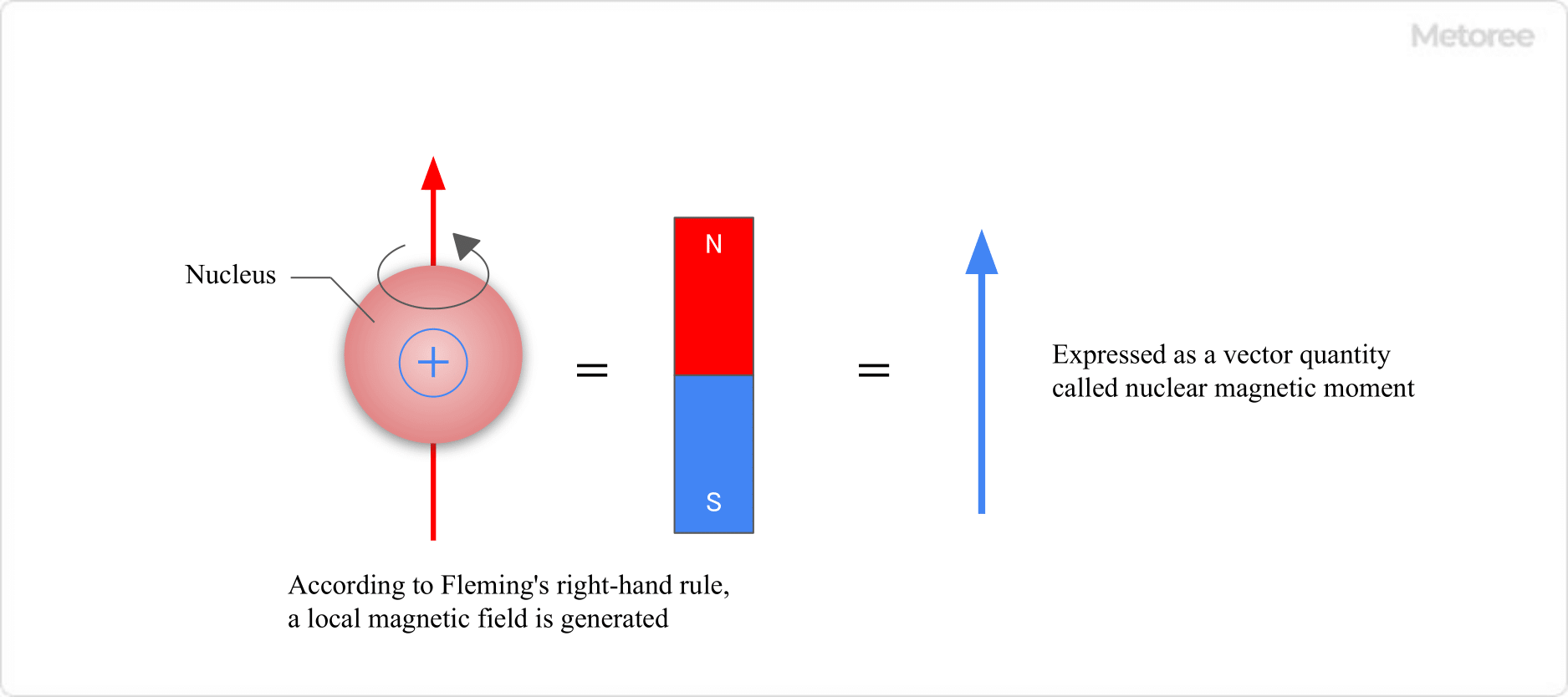
Figure 2. Nuclear Magnetic Moment
An atom’s nucleus, positively charged and spinning, generates a magnetic field, effectively making each atom a small magnet. This field’s magnitude is the nuclear magnetic moment.
2. Zeeman Splitting and Resonance
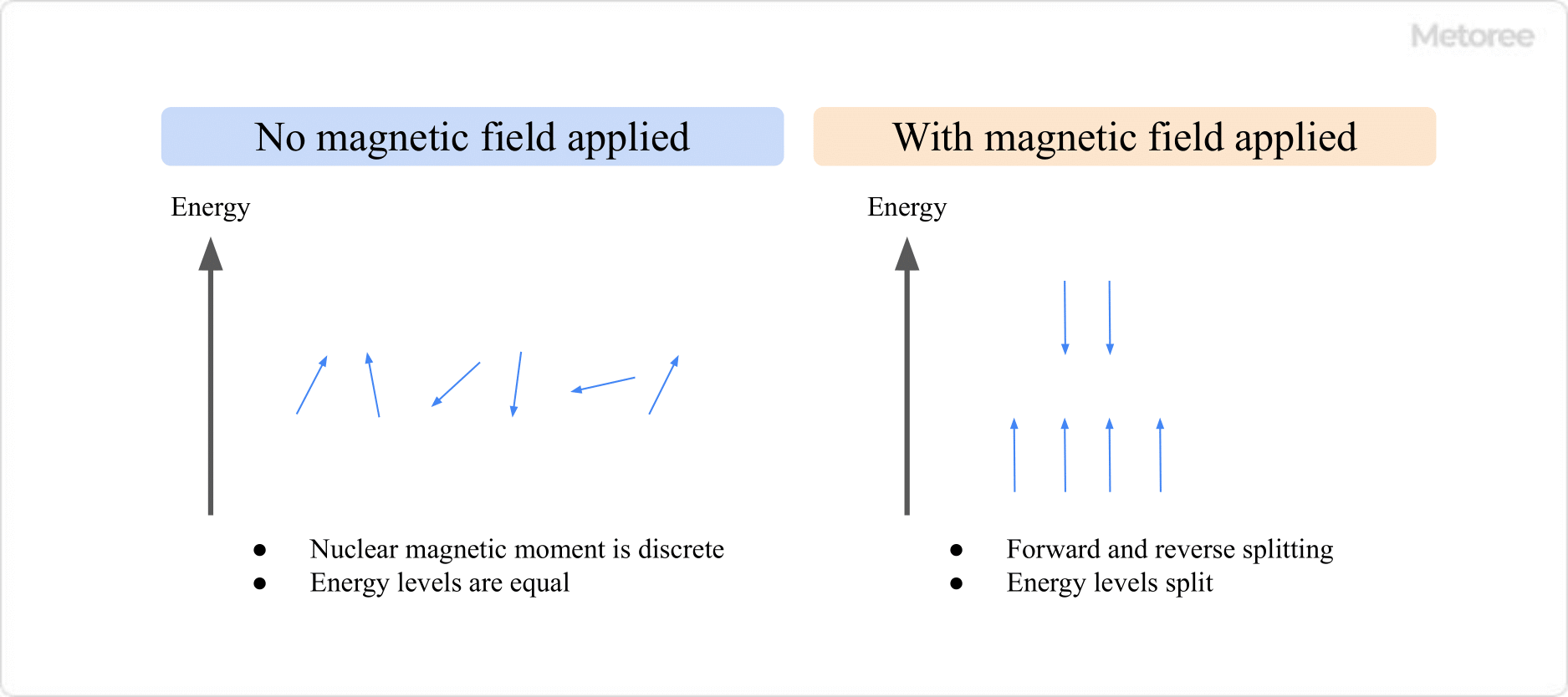
Figure 3. Zeeman Splitting
In a strong magnetic field, nuclei split into two energy states, a phenomenon known as Zeeman splitting. Resonance occurs when electromagnetic waves matching the energy gap induce transitions, enabling the identification of atomic environments.
Zeeman splitting allows observation of varied nuclear compositions, excluding atoms like 12C and 16O, which lack nuclear magnetic moments due to their even mass and atomic numbers.
3. Chemical Shift
Resonance frequencies vary with the nucleus’s environment, measured as the chemical shift in ppm from the reference material’s resonance frequency.
Other Information on NMR Analyzers
NMR Analyzer Cautions
NMR analyzers’ strong magnetic fields attract metal objects and may damage pacemakers, credit cards, and smartphones. The magnets, cooled with liquid helium, require careful management to prevent asphyxiating gas release during abrupt temperature rises.