What Is Thymine?
Thymine, a pyrimidine derivative, is one of the fundamental components of DNA.
DNA comprises four nucleotides: adenine (A), guanine (G), cytosine (C), and thymine (T), with thymine forming two hydrogen bonds with adenine.
Under ultraviolet light, DNA can mutate, forming dimers between adjacent thymine or cytosine bases. Thymine, along with cytosine and uracil, belongs to the pyrimidine base group.
Uses of Thymine
As a DNA component, thymine is crucial for storing the genetic blueprint of living organisms.
In polymerase chain reaction (PCR) testing, used for amplifying and detecting small amounts of genetic material like viruses, thymine is essential. This process requires supplying all four nucleotide types to synthesize a new DNA strand from the extracted gene.
Properties of Thymine
Thymine appears as a white crystalline powder, insoluble in cold water or ethanol but soluble in hot water, hot ethanol, and sodium hydroxide solution.
Oxidation by substances such as oxygen and free radicals can convert thymine into thymine glycol, potentially altering DNA structure. Although antioxidant enzymes and substances repair most cellular and DNA damage, excessive damage may lead to cancer and aging.
Structure of Thymine
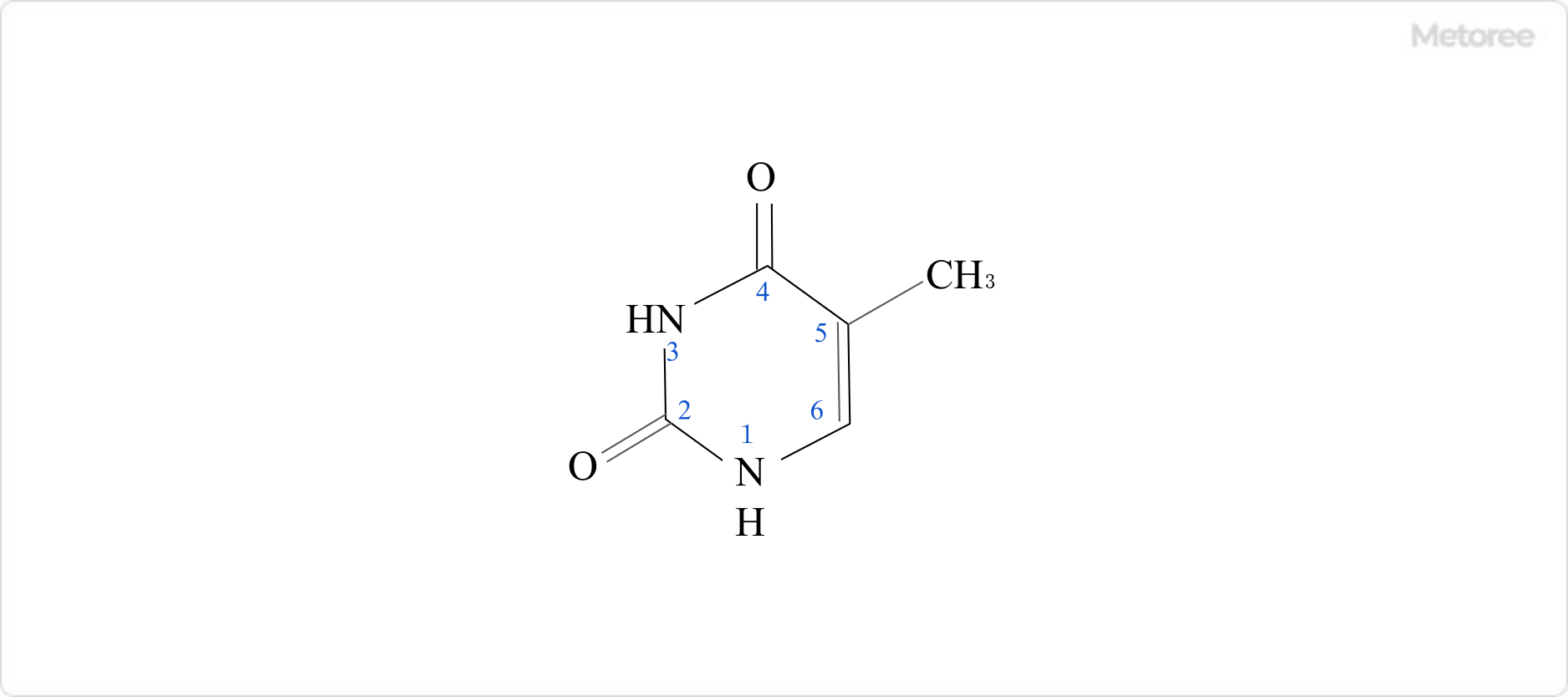
Figure 1. Structural Formula of Thymine
Thymine’s structure includes a pyrimidine ring, characterized by nitrogen-containing six-membered rings, with imino groups at positions 1 and 3, carbonyl groups at positions 2 and 4, and a methyl group at position 5.
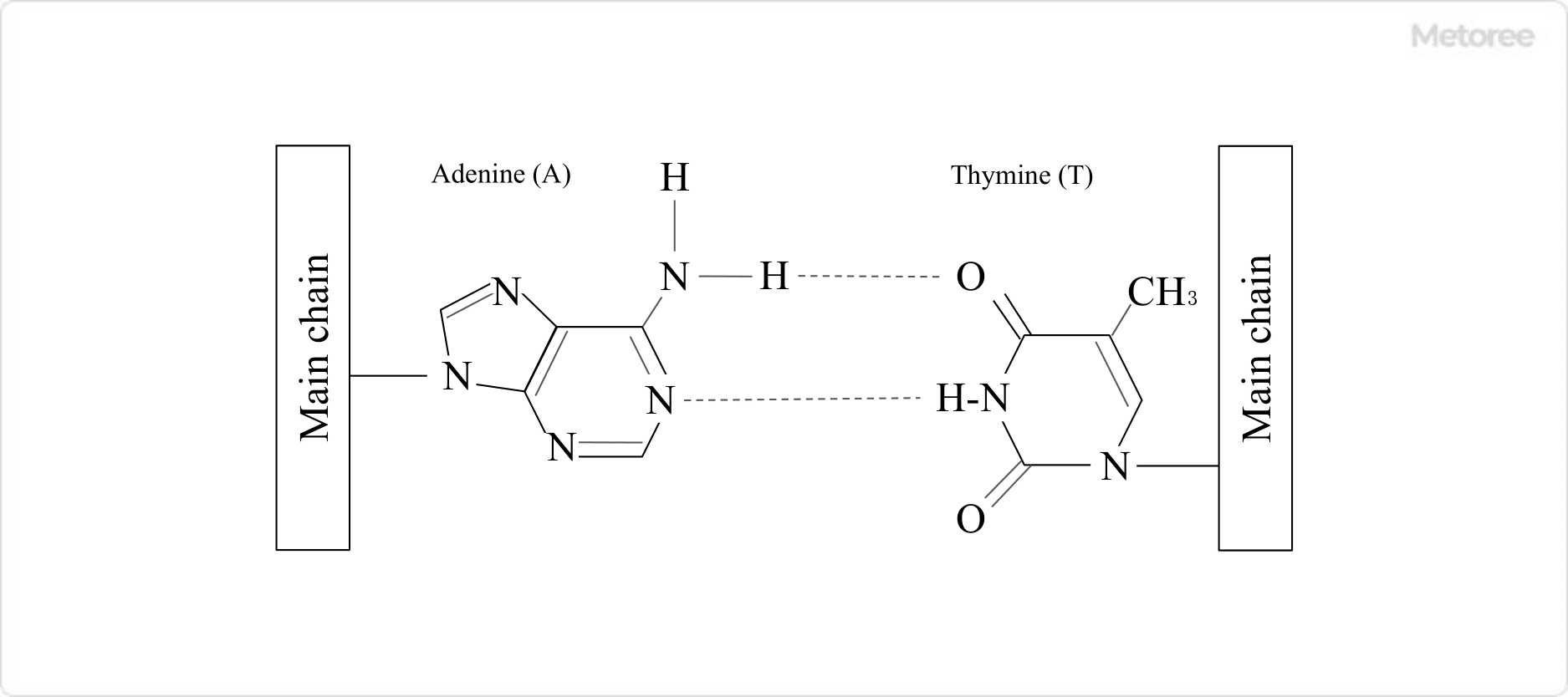
Figure 2. Hydrogen Bonds in DNA
The structure of thymine, particularly its imino and carbonyl groups, is pivotal for DNA’s double helix formation through hydrogen bonding.
Thymine is unique among nucleobases for having a methyl substituent and an allylic hydrogen, making it particularly reactive to oxidative stress in living organisms.
Other Information about Thymine
1. Nucleosides
Nucleosides, which are nucleobase-sugar compounds, include thymidine, formed from thymine and a pentacyclic sugar through a dehydration-condensation reaction involving an N-glycosidic bond.
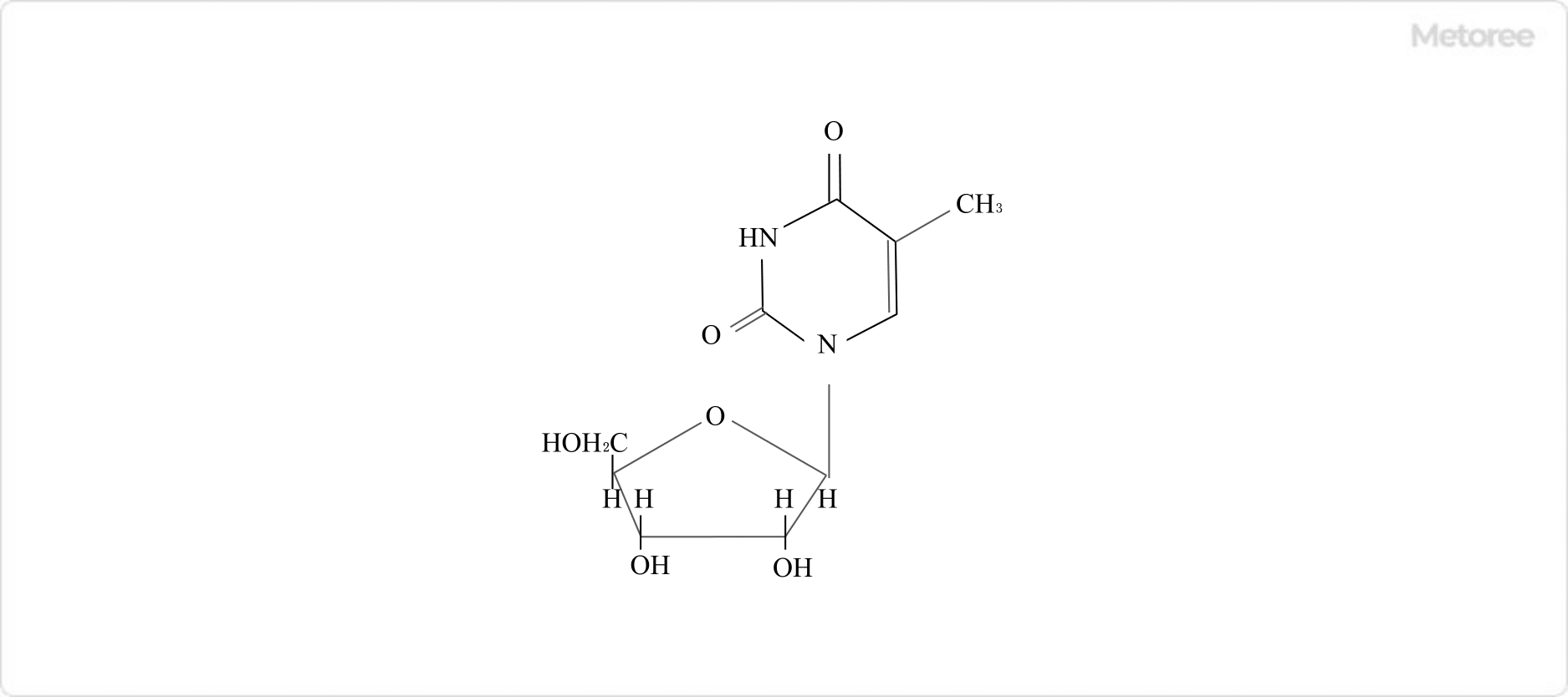
Figure 3. Thymidine
Thymine derivatives include ribothymidine in tRNA and spongothymidine, an anti-herpes agent, synthesized through specific sugar modifications.
2. Nucleotides
Nucleotides, formed by attaching a phosphate group to a nucleoside, include thymidylate or thymidine monophosphate, derived from thymine.
3. Double Helix Structure of DNA
The double helix structure of DNA features hydrophobic bases, including thymine, on the inside with hydrophilic sugars and phosphates on the outside. This structure is stabilized by hydrogen bonds forming base pairs between adenine and thymine, and guanine and cytosine.